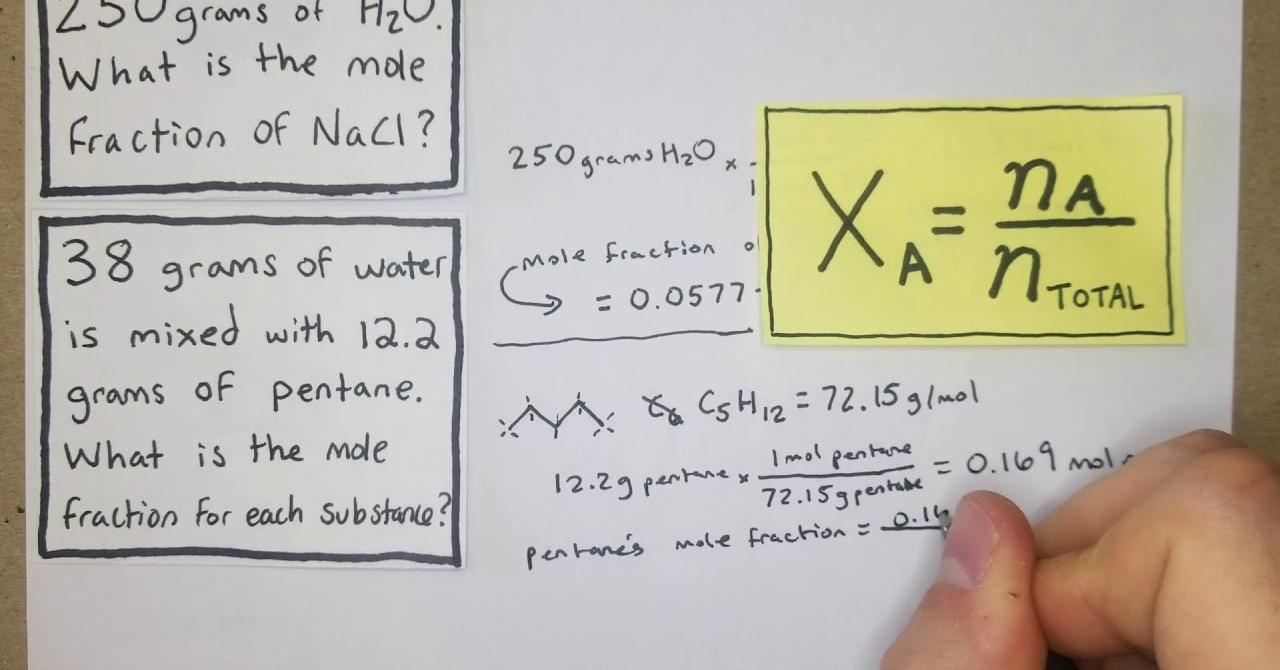Are you curious to know what is mole fraction? You have come to the right place as I am going to tell you everything about mole fraction in a very simple explanation. Without further discussion let’s begin to know what is mole fraction?
Understanding chemical composition is vital in the realm of chemistry, and mole fraction stands as a fundamental concept in expressing the proportion of a component within a mixture. This article aims to demystify the intricacies of mole fraction, offering a comprehensive guide for both students and enthusiasts.
What Is Mole Fraction?
Mole fraction, denoted by the symbol ‘χ,’ represents the ratio of the moles of a specific component to the total moles in a mixture. It provides a quantitative measure of the presence of a substance in a given solution.
What Is The Mole Fraction X Of Solute And Molality?
In the context of solutes and molality, the mole fraction (X) of a solute is calculated by dividing the moles of the solute by the total moles of the solution. This relationship is pivotal in understanding the concentration of substances in a solution.
What Is Mole Fraction In Chemistry? Unveiling Chemical Proportions
In the realm of chemistry, mole fraction plays a crucial role in expressing the concentration of individual components within a mixture. It offers a precise way to communicate the proportional contribution of each substance to the overall composition.
The mole fraction formula is straightforward: χ = moles of component / total moles in the mixture. This formula provides a quantitative measure, ranging from 0 to 1, where 0 signifies the absence of the component, and 1 indicates that the entire mixture comprises that component.
What Is Mole Fraction Example: Practical Applications
Consider a scenario where a solution contains two substances, A and B. If substance A contributes 3 moles, and substance B contributes 2 moles, the mole fraction of A is 3 / (3 + 2) = 0.6, and the mole fraction of B is 0.4.
What Is Mole Fraction And Molar Fraction: Bridging Concepts
Mole fraction and molar fraction are often used interchangeably. Both represent the ratio of moles of a component to the total moles in a mixture. The terms are closely related and are foundational in expressing concentrations in various chemical contexts.
Mole Fraction Unit: Embracing Standard Measurement
The unit of mole fraction is dimensionless, as it is a ratio of moles. This simplicity enhances its applicability and ease of integration into chemical calculations.
Visit Singerbio and read everything about the singer.
Mole Fraction Si Unit: Standardizing Measurement Systems
The mole fraction adheres to the International System of Units (SI), aligning with the global standard for scientific measurement. This standardization ensures consistency and facilitates communication among scientists worldwide.
Mole Fraction Of Gas Formula: Exploring Gas Mixtures
In gas mixtures, the mole fraction of a specific gas is determined by dividing the moles of that gas by the total moles of all gases in the mixture. This formula is invaluable in understanding the behavior of gases in various environments.
How To Find Mole Fraction Of A Gas From Partial Pressure: Practical Steps
Determining the mole fraction of a gas from partial pressure involves using Dalton’s Law. The mole fraction of a gas is equal to the partial pressure of that gas divided by the total pressure of the mixture.
Conclusion:
In conclusion, mole fraction stands as a pivotal concept in chemistry, providing a quantitative means to express the concentration of components within a mixture. From its mathematical representation to practical applications, understanding what is a mole fraction opens doors to unraveling the intricate world of chemical compositions and proportions. Whether delving into solubility studies, gas behavior, or broader chemical analyses, the mole fraction serves as a foundational tool for scientists and students alike.
FAQ
What Is A Mole Fraction In Chemistry?
What is Mole fraction? Mole fraction represents the number of molecules of a particular component in a mixture divided by the total number of moles in the given mixture. It’s a way of expressing the concentration of a solution. Mole Fraction formula.
What Is The Relationship Between Mole Fraction And Concentration?
Whereas mole fraction is a ratio of amounts to amounts (in units of moles per moles), molar concentration is a quotient of amount to volume (in units of moles per litre). Other ways of expressing the composition of a mixture as a dimensionless quantity are mass fraction and volume fraction are others.
What Is Mole Fraction And Mole Percent?
Mole Fraction = the number of moles of one ingredient in the given mixture the total number of moles in the mixture . As a result, Mole percent Equals Mole fraction x 100. Mole percent = the number of moles of one ingredient in the given mixture the total number of moles in the mixture × 100.
Why Do We Use Mole Fractions?
The molar fraction makes it very simple to calculate the mole percent. You can easily calculate the mole percent by multiplying the fraction by 100. You can also get the molar fraction by dividing the mole percent by 100. The molar fraction can be used to determine the concentration of any solute in any given solution.
I Have Covered All The Following Queries And Topics In The Above Article
What Is A Mole Fraction
What Is The Mole Fraction
What Is The Mole Fraction X Of Solute And Molality
What Is Mole Fraction In Chemistry
What Is Mole Fraction Formula
What Is Mole Fraction Example
What Is Mole Fraction And Molar Fraction
Mole Fraction Unit
Mole Fraction Si Unit
Mole Fraction Of Gas Formula
How To Find Mole Fraction Of A Gas From Partial Pressure
What Is Mole Fraction